Home - Cell Line Development - Monoclonality Assurance

SMARTER WORKFLOWS FOR FUTURE MEDICINES
Solentim provides streamlined workflows for the creation, isolation and characterisation of high value cells. For over a decade, Solentim has enabled workflows, validated through Regulatory submission, in key cell therapy areas including cell line development, vaccines, stem cells and gene therapy.
Solentim’s unrivalled VIPS PRO™ and Cell Metric X® instruments work to provide high efficiency single cell seeding and to capture evidence and provide assurance of clonality.
Solentim accelerates cell line workflow timelines from upstream development through scale-up to production of new biological therapies. From vector design and host cell selection prior to transfection, single-cell cloning and colony outgrowth right through to the establishment of the Master Cell Bank, Solentim technologies provide the evidence for credible IND submissions.
DOWNLOAD BROCHURES FOR MORE INFORMATION
The Solentim ecosystem
Workflows for IND success
Assurance of clonality

GENERATING CELL LINES FOR BIOLOGICS MANUFACTURE
Cell line development for biotherapeutics
Enhanced cell line development workflows for successful IND submissions
Solentim’s technologies enable you to generate stable cell lines that create producing novel recombinant proteins, such as monoclonal antibodies or therapeutic proteins for rare diseases.
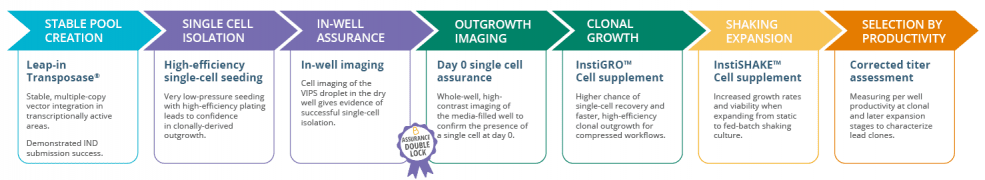
Stable pool creation with Leap-In Transposase® substantially reduce in the number of wells needed to achieve the target number of high expressing clones. Solentim’s unrivalled VIPS PRO and Cell Metric X instruments work to provide high efficiency single cell seeding and to capture evidence and provide assurance of clonality. InstiGRO™ and InstiSHAKE™ Cell Growth Supplements provide high-efficiency clonal outgrowth and increased growth rates and viability when expanding, for compressed workflows.
Development of commercial manufacturing cell lines
Vaccine production with mammalian cells
The use of stable producer HEK293 cell lines for viral vector manufacturing
Solentim’s technologies enable you to isolate, screen and characterise attributes for clones producing novel recombinant sub-unit protein and monoclonal antibody vaccines.
Stable cell lines are required for industrial production of recombinant protein vaccines. These cell lines must have the ability to produce the same high-quality products at different times, at different geographical sites and between different batches.
The workflow steps for stable cell lines for vaccine production are very similar to those outlined in the Biotherapeutics section. Current best practice with immortalised cell lines producing a vaccine is to perform single-cell isolation with systems, such as the VIPS PRO and to monitor clonal outgrowth with the Cell Metric X. The history of the clone is then documented by way of the Clonality Report which can be submitted to the regulator.
Engineered exosome producer cell lines
Making master cell banks for stable exosome producer cell lines
Solentim’s technologies enable you to establish stable producer cells for the production of engineered and cell-engineered exosomes.
Exosomes are a rising star in the world of therapeutics. With unique surface markers, exosomes have the potential to seek and deliver their payload to specific tissue types or disease states. As with other biologics, stable cell lines are required for industrial production of exosomes and these cell lines must have the ability to produce the same high-quality products batch-to-batch.
The workflow steps for stable cell lines for exosome production are very similar to those outlined in the Biotherapeutics section. Solentim’s unrivalled VIPS PRO and Cell Metric X instruments work to provide high efficiency single cell seeding and to capture evidence and provide assurance of clonality. InstiGRO and InstiSHAKE Cell Growth Supplements provide high-efficiency clonal outgrowth and increased growth rates and viability when expanding, for compressed workflows.

GENERATING CELL LINES FOR CELL AND GENE THERAPY
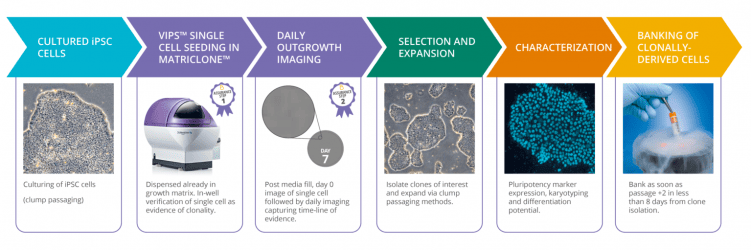
Efficient cloning of hiPSCs
Single cell cloning of hiPSCs
Supporting future manufacturing of stem cell therapies
Solentim’s technologies enable high efficiency, high viability single cell cloning of hiPSCs for applications including development of disease models and supporting GMP manufacture of cell therapies.
Due to their inherent nature, iPSC single cell cloning presents challenges to the stem cell community leading many to use time consuming manual seeding methods and inconsistent coating-plating plates protocols.
Solentim has introduced a novel and robust workflow for the single cell cloning of hiPSCs using the VIPS PRO in combination with a new soluble dispensing matrix called MatriClone™.
VIPS PRO offers high efficiency seeding of high viability cells and dispenses MatriClone as part of the seeding process. MatriClone is a defined animal component-free matrix and facilitates cell attachment and growth without the need for pre-coating culture plates. MatriClone’s optical qualities is perfectly suited to enable VIPS PRO to capture high quality evidence of clonality.
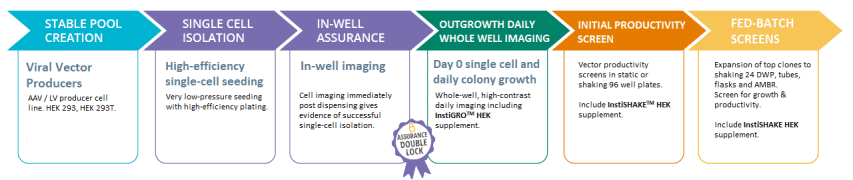
Gene therapy vector production
The use of stable producer HEK293 cell lines for viral vector manufacturing
From the selection of high producer clones to the development of stable vector producer cell lines, explore Solentim’s simplified workflows for gene therapy applications.
Solentim’s unrivalled VIPS PRO and Cell Metric X instruments work to provide high efficiency single cell seeding and to capture evidence and provide assurance of clonality. InstiGRO and InstiSHAKE Cell Growth Supplements provide high-efficiency clonal outgrowth and increased growth rates and viability when expanding, for compressed workflows.
Improved clonal outgrowth of stable vector producing cell lines

GENERATING CELL LINES FOR DRUG DISCOVERY
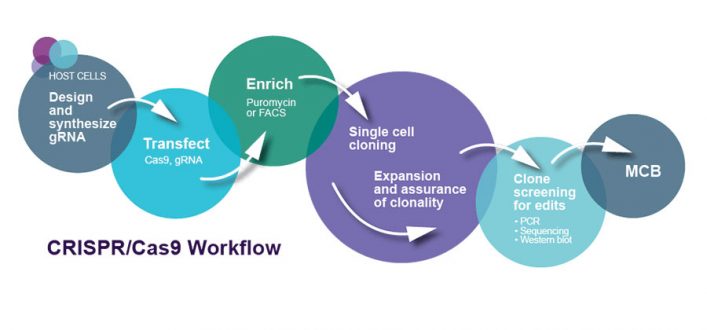
Cell engineering and precision gene editing
Target identification and validation in the drug discovery workflow
Optimise your drug discovery process using targeted gene editing to create stable cell lines as cellular reagents, for use in target validation, functional genomics and disease modelling.
For scientists in early-stage drug discovery, working with CRISPR pools is a high-throughput approach for initial target identification or for selecting a phenotype. For target validation and unravelling disease pathways and cellular processes, gene knock-outs are required for each gene and these need to be in stable cell lines.
Solentim’s unrivalled VIPS PRO and Cell Metric X instruments work to provide high efficiency single cell seeding and to capture evidence and provide assurance of clonality. To meet the high-throughput requirements of this workflow, customers can either use the Cell Metric X HT with incubated plate load and cassette for walkaway screening of 10 plates, or the Cell Metric X R can be linked through an API to a third-party robotic arm.

Case Studies
DOWNLOAD BROCHURES FOR MORE INFORMATION
The Solentim ecosystem
Workflows for IND success
Assurance of clonality